

TEQs - A Handy Regulatory Tool
I am intrigued by the process of finding solutions to environmental problems, especially when they relate in a practical way to human health and biological/chemical interactions within the body. Often, the field of environmental chemistry is viewed as a discipline that many nonscientists shy away from because the study of chemistry has a reputation for being difficult, but sometimes these complex scientific ideas can be explained in ways that everyday people can easily understand.
In all my experiences collaborating with engineers and regulators at both the federal and state levels, one of my favorite topics to work with during the last 15 years is toxic equivalency quotients (TEQs). This handy regulatory tool allows environmental cleanup experts to focus analytical efforts on obtaining a grid of sample concentrations of one known carcinogen while also capturing knowledge of a wider analytical breadth of compounds or potential carcinogens.
This analytical strategy is often used for projects involving carcinogenic polyaromatic hydrocarbons (PAHs). This group includes seven well-researched compounds that have the potential to alter their chemical structure and eventually morph into a deadly compound, benzo(a)pyrene, which is infamous in the toxicological field for being an agent that can cause a mutation in the structure of DNA and lead to cancerous byproducts in the cell. There are several PAHs that contribute to cell mutation in other indirect ways, but the active site mechanism for benzo(a)pyrene is circled below in the image below. The other six carcinogenic PAH sister-compounds have varying propensities for degrading or changing into the structure of benzo(a)pyrene. They behave like chemical chameleons entering the cell, getting by the gatekeeper, then changing color (or in this case morphing) as time goes on once inside the cell.
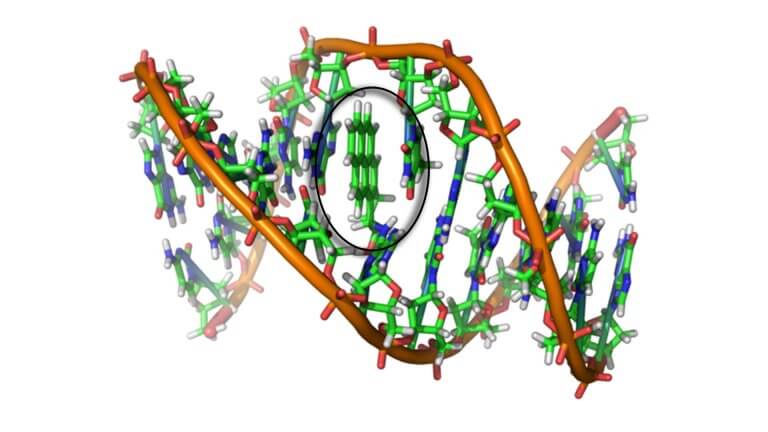
There are several PAHs that contribute to cell mutation in other indirect ways, but the active site mechanism for benzo(a)pyrene is circled in the image.
With the enhancements of the selective ion monitoring (SIM) technology, SW-846 Method 8270 allows Chemists to selectively tune the mass spectrometer to focus on obtaining only the concentrations of these seven PAHs and, once collected, assign a weighting, which uses a reference weighting of ‘1’ for benzo(a)pyrene. The other six compounds have individual weightings of varying orders of magnitude less than ‘1’ (i.e., 0.1, 0.01, 0.001). As these new weighted concentrations are summed up, the Chemist is allowed to consolidate the data and come up with one number: the TEQ.
To describe this in a way that everyday people can easily visualize and understand, imagine that we were comparing pieces of fruit with respect to volume. Since watermelon is huge, let’s give that a value of 1 and make it our reference. Peaches are much smaller, and we can assign them a weighting of .065. If we add cherries, which are even smaller, maybe we would assign a weighting of about .003 for them. So, if we were testing a group that had: 1 watermelon, 11 peaches, and 100 cherries, then the total fruit-volume TEQ of this sample would be 2, because the total volume of all these different fruits together is similar to the volume of two watermelons.
TEQs can also be used for other types of carcinogens analyzed using different methods, such as the family of dioxin-furan compounds. In this scenario, the Chemist is typically analyzing for a list of 17 compounds, or congeners, that are all weighted on a refence scale that uses 2,3,7,8-tetra-chlorodibenzo-p-dioxin (TCDD) as the reference compound. Regardless of the toxicological family of compounds in question, the fundamental idea of the TEQ remains the same. The ‘weighting,’ or toxic equivalency factor (TEF) is a constant that is specific to each molecule that is used to be multiplied by each positive result and expresses the toxicity of the entire group in terms of the most toxic form of each group: benzo(a)pyrene in the case of PAHs or 2,3,7,8-TCDD in the case of dioxin-furans.
This is fine, but what does one do for compounds that are “not-detected” (ND) in a given sample? Or worse yet, what does one do when all the compounds are ND in each sample? Throughout different state and national agencies, there are varying remedies for handling situations for which there are one or more of the chemical group compounds that are ND for a given sample. Often, flow charts and guidance documents created by agencies for these cases; giving specific instructions to be followed in order to correctly assess whether the detection limit, reporting limit, or any Quality Assurance Project Plan- (QAPP-) provided quantitation limit will be used in place of a positive value to be, in turn, multiplied by the TEF and taken into the summation of the TEQ. While it is common for parties to agree on using 0.5 as a place to begin the project, there are often situations that come into play for which it may be acceptable to use “0” in place of a detection limit if that particular compound has never been detected in a particular matrix at the site in question. Conversely, there may be situations for which “1” may be used if a particular compound has been notoriously high at the site of concern.
Example of TEFs of Standard TEQ methodology
CAS Number | Hazardous Substance | TEF1 |
Dioxin Congeners | ||
1746-01-6 | 2,3,7,8-Tetrachloro dibenzo-p-dioxin (TCDD) | 1 |
40321-76-4 | 1,2,3,7,8-Pentachloro dibenzo-p-dioxin (PCDD) | 1 |
39227-28-6 | 1,2,3,4,7,8-Hexachloro dibenzo-p-dioxin (HxCDD) | 0.1 |
57653-85-7 | 1,2,3,6,7,8-Hexachloro dibenzo-p-dioxin (HxCDD) | 0.1 |
19408-74-3 | 0.1 | |
35822-46-9 | 0.01 | |
3268-87-9 | 1,2,3,4,6,7,8,9-Octachloro dibenzo-p-dioxin (OCDD) | 0.0003 |
Furan Congeners | ||
51207-31-9 | 2,3,7,8-Tetrachloro dibenzofuran (TCDF) | 1 |
57117-41-6 | 1,2,3,7,8-Pentachloro dibenzofuran (PCDF) | 0.03 |
57117-31-4 | 2,3,4,7,8-Pentachloro dibenzofuran (PCDF) | 0.3 |
70648-26-9 | 1,2,3,4,7,8-Hexachloro dibenzofuran (HxCDF) | 0.1 |
57117-44-9 | 1,2,3,6,7,8-Hexachloro dibenzofuran (HxCDF) | 0.1 |
72918-21-9 | 0.1 | |
60851-34-5 | 2,3,4,6,7,8-Hexachloro dibenzofuran (HxCDF) | 0.1 |
67562-39-4 | 1,2,3,4,6,7,8-Heptachloro dibenzofuran (HpCDF) | 0.01 |
55673-89-7 | 1,2,3,4,7,8,9-Heptachloro dibenzofuran (HpCDF) | 0.01 |
39001-02-0 | 1,2,3,4,6,7,8,9-Octachloro dibenzofuran (OCDF) | 0.0003 |
(1) Source: Van den Berg, et al. (2006) and Van den Berg et al. (1998). The 2005 World Health Organization re-evaluation of human and mammalian toxicity equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences, 2006 93(2), 223-241; doi:10.1093/toxics/kfl055.
Dioxin TEQ calculation using ½ the Estimated Detection Limits (EDLs) for NDs
2,3,7,8-TCDD………………………………………………..…14 ppt (1.0) *1 = 14
1,2,3,7,8-PCDD………………………………………………….5 ppt (1.0) *1 = 5
1,2,3,4,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
1,2,3,6,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
1,2,3,7,8,9-HxCDD……………………………………………20 ppt (2.0) *0.1 = 2.0
1,2,3,4,6,7,8-HpCDD……………………………………………..ND (2.0) *0.01 = 0.01
1,2,3,4,6,7,8,9-OCDD……………………………………….55 ppt (5.0) *0.0003 = 0.0165
2,3,7,8-TCDF……………………………………………………15 ppt (1.0) *1 = 15
1,2,3,7,8-PCDF…………………………………………………..4 ppt (1.0) *0.3 = 1.2
2,3,4,7,8-PCDD……………………………………………………..ND (2.0) *0.3 = 0.3
1,2,3,4,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
1,2,3,6,7,8-HxCDD……………………………………………20 ppt (2.0) *0.1 = 2.0
1,2,3,7,8,9-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
2,3,4,6,7,8-HxCDD……………………………………………55 ppt (5.0) *0.1 = 5.5
1,2,3,4,6,7,8-HpCDD……………………………………..…20 ppt (2.0) *0.01 = 0.2
1,2,3,4,7,8,9-HpCDD……………………………………..………ND (2.0) *0.01 = 0.01
1,2,3,4,6,7,8,9-OCDD……………………………………….60 ppt (5.0) *0.0003 = 0.018
Final TEQ = 45.7
Dioxin TEQ calculation using ND = 0
1,2,3,6,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
1,2,3,7,8,9-HxCDD……………………………………………20 ppt (2.0) *0.1 = 2.0
1,2,3,4,6,7,8-HpCDD……………………………………………..ND (2.0) *0.01 = 0.01
1,2,3,4,6,7,8,9-OCDD……………………………………….55 ppt (5.0) *0.0003 = 0.0165
2,3,7,8-TCDF……………………………………………………15 ppt (1.0) *1 = 15
1,2,3,7,8-PCDF…………………………………………………..4 ppt (1.0) *0.3 = 1.2
2,3,4,7,8-PCDD……………………………………………………..ND (2.0) *0.3 = 0.3
1,2,3,4,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
1,2,3,6,7,8-HxCDD……………………………………………20 ppt (2.0) *0.1 = 2.0
1,2,3,7,8,9-HxCDD…………………………………………………ND (2.0) *0.1 = 0.1
2,3,4,6,7,8-HxCDD……………………………………………55 ppt (5.0) *0.1 = 5.5
1,2,3,4,6,7,8-HpCDD……………………………………..…20 ppt (2.0) *0.01 = 0.2
1,2,3,4,7,8,9-HpCDD……………………………………..………ND (2.0) *0.01 = 0.01
1,2,3,4,6,7,8,9-OCDD……………………………………….60 ppt (5.0) *0.0003 = 0.018
Final TEQ = 45.7
Dioxin TEQ calculation using ND = 0
2,3,7,8-TCDD………………………………………………..…14 ppt (1.0) *1 = 14
1,2,3,7,8-PCDD………………………………………………….5 ppt (1.0) *1 = 5
1,2,3,4,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0
1,2,3,6,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0
1,2,3,7,8,9-HxCDD……………………………………………20 ppt (2.0) *0.1 = 2.0
1,2,3,4,6,7,8-HpCDD……………………………………………..ND (2.0) *0.01 = 0
1,2,3,4,6,7,8,9-OCDD……………………………………….55 ppt (5.0) *0.0003 = 0.0165
2,3,7,8-TCDF……………………………………………………15 ppt (1.0) *1 = 15
1,2,3,7,8-PCDF…………………………………………………..4 ppt (1.0) *0.3 = 1.2
2,3,4,7,8-PCDD……………………………………………………..ND (2.0) *0.3 = 0
1,2,3,4,7,8-HxCDD…………………………………………………ND (2.0) *0.1 = 0
1,2,3,6,7,8-HxCDD……………………………………………20 ppt (2.0) *0.1 = 2.0
1,2,3,7,8,9-HxCDD…………………………………………………ND (2.0) *0.1 = 0
2,3,4,6,7,8-HxCDD……………………………………………55 ppt (5.0) *0.1 = 5.5
1,2,3,4,6,7,8-HpCDD……………………………………..…20 ppt (2.0) *0.01 = 0.2
1,2,3,4,7,8,9-HpCDD……………………………………..………ND (2.0) *0.01 = 0
1,2,3,4,6,7,8,9-OCDD……………………………………….60 ppt (5.0) *0.0003 = 0.018
Final TEQ = 44.9
As demonstrated by the bold values above, deciding whether we use 0, 0.5, or 1 as a multiplier for the EDL will lead to differences in the outcome. In this example, with a regulatory risk value of 45, these two variations of the standard method of substitution could lead to the difference between a sample being above or below the risk value. The question of what percentage, if any, of the detection limit should be used in place of a compound that is ND is not without controversy. The high degree of variability in data streams using one percentage or another can make evaluating site history quite challenging – even though the same analytical method may have been used for large amounts of data, they are oftentimes not comparable simply because different variations of the TEQ methodology may have been used over time.
Is there a more consistent way to provide comparable data streams in these cases?
Tune in for our next quarterly newsletter in December, and I will explain the statistical mechanism behind an incredible tool that can work very well for these situations known as the Kaplan-Meier method.

