
Hydrocarbon Forensics:
Cracking the Code
Forensic Chemists are experts at solving environmental mysteries and puzzles. One of the most complex challenges we tackle is hydrocarbon contamination. This contamination can stem from sources including fuel releases, crude oil spills, natural seeps, residues from manufactured gas plants (MGPs), background contamination from air deposition, and plant products.
To identify the origins of contamination, Forensic Chemists use chemical characteristics to identify and differentiate the sources. In this article, we explore the methods and chemical markers we use to crack the code.

Fuel Releases such as gasoline, diesel, and jet fuel, are frequently encountered. These refined products have specific chemical compositions. Key markers include:
- Alkanes and Aromatics: We often see recognizable mixtures of alkanes (paraffins) and aromatic hydrocarbons. Gasoline, for example, is rich in light alkanes (C4-C12) and benzene, toluene, ethylbenzene, and total xylenes (BTEX). Substituted monoaromatics, alkylates, and naphthenic compounds help us characterize the particular refinery formulation.
- Additives: Organoleads, methyl tert-butyl ether (MTBE), and ethanol each had production peak year ranges.
- PAHs: Polycyclic aromatic hydrocarbons (PAHs) are found more in diesel and jet fuels than gasoline. Specific PAH profiles help us identify different types of fuel releases.
- Alkylated PAHs (APAHs): These are major components of crude oils and heavier fuels. They are reported as homolog totals (e.g., C1-phenanthrenes, C2-phenanthrenes, etc.) and then plotted as histograms for profiling – very useful for fuels heavier than gasoline.
Crude Oil Releases are complex mixtures, varying by source. Key chemical markers include:
- Alkanes and Isoprenoids: Crude oil contains a wide range of alkanes, including n-alkanes and branched alkanes like pristane and phytane. The ratios of these compounds can provide information about the oil’s source and weathering state.
- APAHs: Crude oils contain greater loadings of APAHs than parent PAHs. Crude oil (petrogenic) APAH profiles are clearly distinguishable from (thermogenic) residues.
- Biomarkers: Compounds such as steranes and hopanes are derived from biological precursors and resist degradation. Biomarkers can be used to distinguish between oils (and fuels) produced from different geologic formations.
- PAHs: We use PAH diagnostic ratios to distinguish crude oils from each other and from non-petroleum hydrocarbon sources, and they’re important for the following application.
MGP Residues were produced from coal, oil, or other feedstocks, leaving behind complex residues with distinctive profiles.
- Coal Tar: Coal tar MGP residue is rich in PAHs, particularly heavier PAHs and APAHs. Compounds like benzo[a]pyrene and chrysene can indicate MGP residues. Diagnostic ratios of PAHs and APAHs distinguish coal tar from oil residue.
- Phenolic Compounds: MGP residues contain phenolic compounds more commonly than other hydrocarbon sources. We also use these markers to identify MGP contamination.
- Cyanides and Sulfur Compounds: Help us distinguish MGP residues from other contamination sources.
Background Hydrocarbon Contamination can arise from the following sources:
- Air Deposition: Hydrocarbons from air deposition can include a mix of combustion byproducts, such as PAHs from vehicle emissions and industrial activities. The PAH profiles from air deposition are often more complex and include a wider range of compounds compared to point-source contamination.
- Plant-produced: Hydrocarbons such as terpenes and waxes are distinguished from petroleum hydrocarbons by their specific chemical structures.
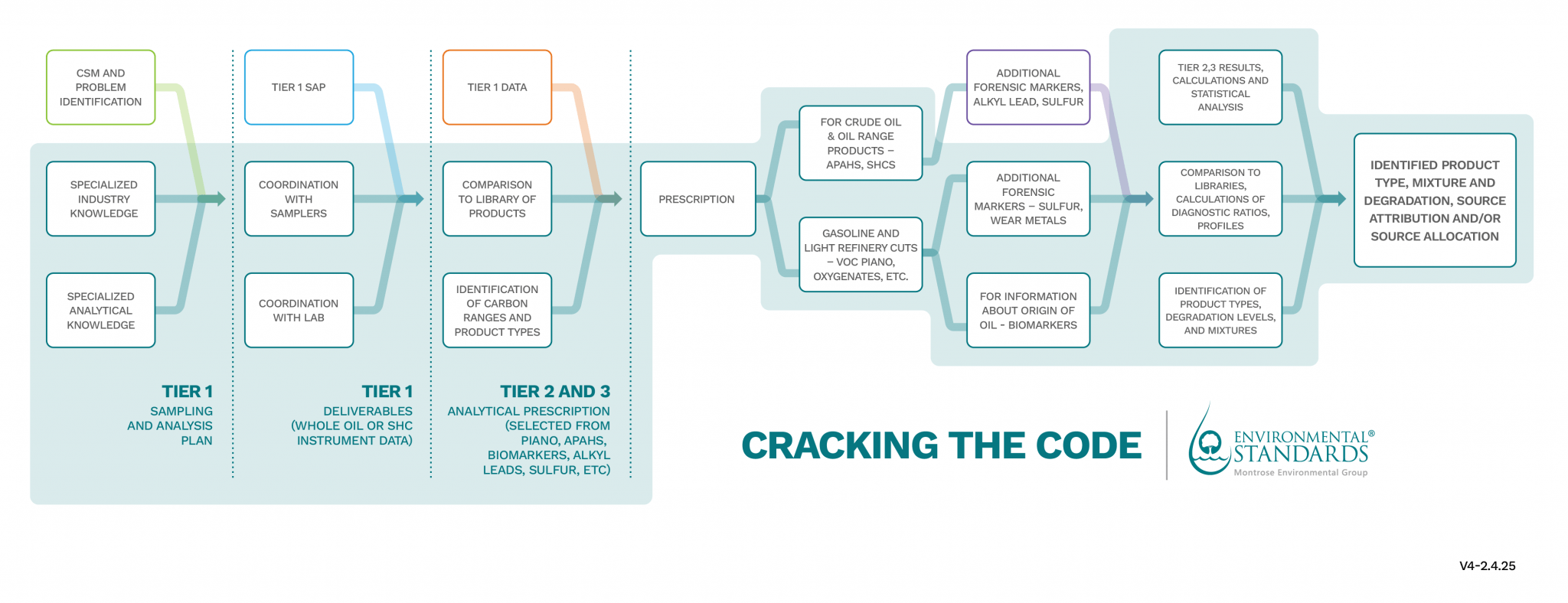
Analytical Techniques we use to identify and quantify hydrocarbons from different sources include:
- High-Resolution Gas Chromatography-Flame Ionization Detection: This technique is widely used as a first step (or “Tier 1”) analysis to, at a glance and through comparison to library reference chromatograms, identify the range of hydrocarbons and degree of degradation that the hydrocarbons have undergone. This can be done through whole oil analysis that can capture profiles ranging from C4-C44, or if an extraction is necessary, saturated hydrocarbon analysis that gives quantified results for C8-C40 alkanes and selected biomarkers.
- Chromatography-Mass Spectrometry (GC-MS): This technique is widely used to analyze complex mixtures of hydrocarbons. It can separate and identify individual compounds, providing detailed chemical profiles. Based on the results of the Tier 1 analysis, we will select from specialized VOC (PIANO) analysis, PAH/APAH, and biomarker analyses.
- High-Performance Liquid Chromatography (HPLC): HPLC is useful for analyzing non-volatile and thermally labile compounds, such as certain PAHs and phenolic compounds.
- Stable Isotope Analysis: This method can provide information about the origin and transformation of hydrocarbons. Isotopic ratios of carbon and hydrogen can help distinguish between biogenic and petrogenic sources.
The code is cracked when our Forensic Chemists use a combination of chemical markers and analytical techniques to distinguish between different types of hydrocarbon contamination. By understanding the unique chemical signatures of fuel releases, crude oil spills, MGP residues, and background contamination, we can accurately identify and address the sources. Increasingly, we are called upon to use this approach to inform effective remediation, equitable cost sharing, and protection of environmental and human health.
Do you have a challenging hydrocarbon contamination mystery that needs solving? Our experts at Environmental Standards are here to help.

