
When an accredited environmental laboratory reports data, many people’s first instinct is to trust it completely. The laboratory adheres to requirements set by regulators and performs analytical testing according to relevant methods, federal and state regulations, and their own internal standard operating procedures (SOPs). So, what could go wrong?
Environmental Standards has been helping clients answer that question for over 30 years. The most common quality assurance (QA) service provided by Environmental Standards is data validation – a systematic process for reviewing analytical data against established criteria to assess their validity before use. This process shows whether the data meet method requirements, SOPs, and other standards, and if they are of adequate quality. Then a summary of potential usability issues is created. However, this is not the only tool in our “toolkit” for determining if laboratory-reported data are of the highest quality. For some projects, reviewing historical data in conjunction with data validation has uncovered many sample-specific and systematic laboratory issues.
In this article, Project Quality Assurance Chemist Danielle Coles explains the importance of reviewing historical data to compare past data trends against current data, which can help identify sources of error so that corrective measures can be implemented. She demonstrates how confirming the integrity of current data through this process can allow for improved outcomes and better decision-making.
Historical Data Review: When and How?
Historical data review involves comparing reported data to previous analytical results for a specific sampling location. This review is initially conducted separately from the data validation process to avoid bias. Unlike data validation, this review helps identify laboratory issues such as occasional field or laboratory contamination and possible sample switches. Ultimately, laboratory data may be compliant and seem sufficiently high quality, but still not be truly representative of the location where the sample was collected.
Factors to consider when determining if a historical data review will be worthwhile for a new project:
- This process requires a robust dataset (at least 4-5 previous results)
- The review has been most effectively used for routinely monitored aqueous matrices but could be applied to other matrices depending on sampling frequency.
- Sample locations should be consistent (i.e., an installed monitoring well, surface water, or soil samples collected from the same GPS coordinates).
After determining that a project is a good fit for this review, there are several options for how the review will be performed.
- Tabular review: a direct comparison of results to previous ones (analyzing the numbers)
- Historical time series (graphical representation)
- Statistical approach: establishing upper and lower “limits.” This approach could be less manual but might overlook anomalies in the data depending on how the limits are set.
No matter the approach, when evaluating a newly reported dataset, this review might generate a list of “outliers” compared to historical results. Once that list is created, the real investigation can begin.
What Comes Next?
When a historical review surfaces an issue, the decision to take further action requires multiple lines of evidence. A thorough review of the laboratory data package will help determine if there is sufficient evidence to support having the laboratory review the reported data. The key question that needs to be answered is whether the analytical data support an error. Check whether their additional analytical runs or further analyses support or refute the result(s) in question.
Additionally, you may need to evaluate larger seasonal trends. Depending on the availability of previous data, you might want or need to extend the timeline. Reviewing field data can also help determine if it indicates a change in conditions at that location. Field measurements that often provide hints include pH, oxidation-reduction potential (ORP), and specific conductance. It is also recommended to review the sampling personnel’s notes for indications of weather; for groundwater samples, flooding or drought conditions can impact analytical results.
If additional evidence does not help explain the deviation from historical results, the laboratory (and Field Teams if necessary) should be asked to review and confirm the reported data.
The investigation then returns to the source (either the field or laboratory personnel) to ensure their documentation is accurate, reanalyze the sample if they believe it is necessary, and then, depending on what is uncovered, report revised results for the impacted samples. When re-reporting is required, thorough documentation is needed to examine all steps of the process as well as the potential root cause of the issue that impacted the originally reported results. The ultimate goal is always data integrity and accuracy, and sometimes the best way to ensure accuracy is to leverage the data you already have. The following case studies demonstrate the benefits of incorporating historical data review, in addition to data validation, into a QA process.
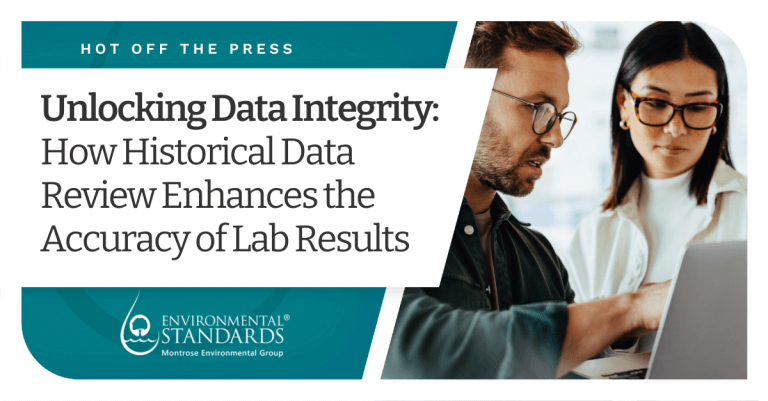
Case Study: Sample Contamination
Chromium data for this sample was significantly higher than previously reported data, and there was no historical evidence to support this large variation for the field duplicate. In this instance, because the issue was with a field duplicate, the parent sample result provided strong evidence of a potential laboratory issue. The laboratory was asked to confirm the reported result and could not. The laboratory decided to reanalyze, and the reanalysis results were reported. The laboratory had several incidents of “one-off” contamination, and it appeared the issue was becoming systemic. A corrective action was issued, and the laboratory improved its cleaning procedures and updated processes to help avoid contamination in the future.
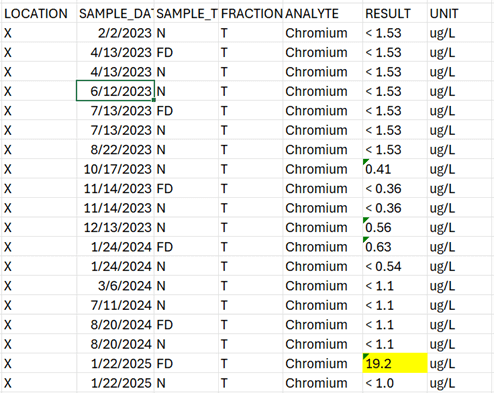
Case Study: Sample Switch at the Lab
The historical data review indicated that a sample switch had occurred during the metals analysis, which swapped the results for samples “Well A” and “Well B.” This issue was not seen in the other analytical fractions (anions, general chemistry) included for these samples. The laboratory was consulted, and after reanalysis, it was confirmed that a sample switch had occurred in the metals laboratory. The reanalysis results were reported to ensure accuracy of results for each well location. Again, formal laboratory corrective action was needed due to the recurrence of sample switches in the same department.
Without historical data review, this issue would not have been apparent because metals data were compliant with the method, and laboratory quality control samples did not indicate any issues.
Your commitment to data accuracy starts here! To learn more about how Data Validation and Historical Data Review can improve your business decision-making and outcomes, reach out to our team of experts today.

